Background:
Acute lymphoblastic leukemia (ALL) is rare but aggressive. For refractory and relapsed ALL (R/R ALL), various treatment strategies are adopted, of which the use of blinatumomab has shown promising results in recent decades. Blinatumomab is a CD-19 directed CD3 bispecific T-cell engager antibody that has been approved by the FDA for the treatment of both adults and pediatric ALL. We report the efficacy and toxicity of blinatumomab in relapsed and refractory ALL adult patients.
Materials/Methods:
Following the PRISMA guideline, we performed a comprehensive literature search PubMed, Embase, Cochrane Library, Web of Science, and Clinicaltrials.gov. We used the following keywords, "acute lymphocytic leukemia" and "Blinatumomab" from the inception of data till 06/17/2020. We screened 1199 articles and included 7 trials and 6 retrospective studies. We excluded all case reports, case series, preclinical trials, review articles, and meta-analysis. We extracted the data for efficacy (complete response, relapse, overall survival, etc.) and safety (≥Grade 3 adverse events).
Results:
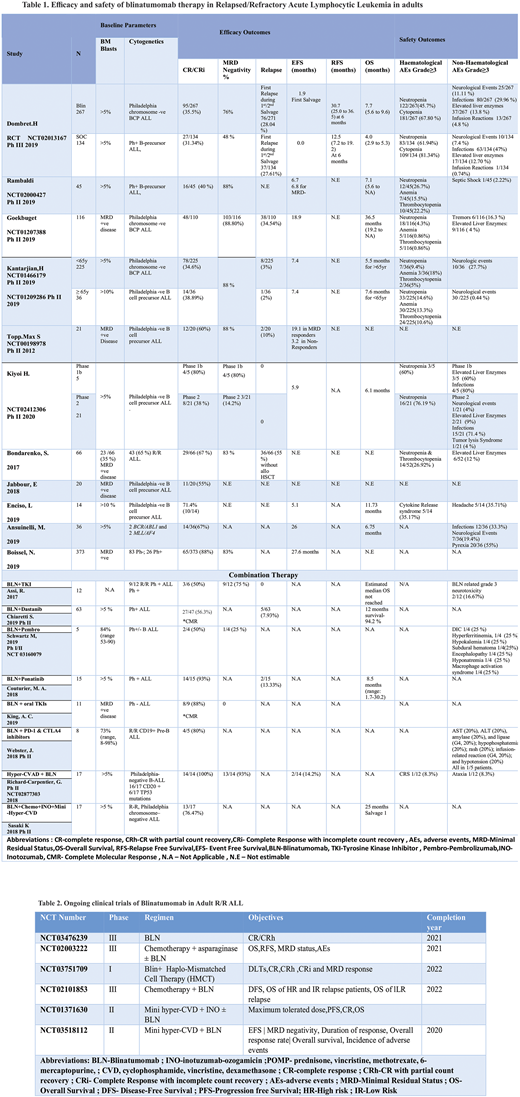
A total of 1393 patients with relapsed/refractory ALL were included.
Monotherapy: In phase 3 TOWER trial, a total of 405 patients with heavily pretreated B-cell precursor ALL were randomized in a 2:1 ratio to either receive blinatumomab (n=271) or standard of care (SOC) (N=134). The mean age of both groups was 40.8 years and 41.1, respectively. The dose of blinatumomab (9-28 ug/d) was instituted to the intervention group, whereas the control group received investigator-chosen chemotherapy regimens. The CR/CRi rate for both groups was 95/267 (35.5 %) and 27/134 (31.34%), respectively (p<0.01). The median overall survival was 7.7 (5.6 to 9.6 months) vs. 4.0 (2.9 to 5.3 months); the hazard rate for death: 0.71 (95% CI 0.55-0.93) p=0.01 and MRD negativity was 76 % and 48 %, respectively. The most common grade 3 and 4 adverse events (AEs) were neutropenia 45.7%) vs. 61.94% and cytopenia 67.80% vs. 81.34% respectively.
In 6 phase 1b/2 studies (N=469), the starting dose of blinatumomab was 5 µg /m2/d and subsequently increased to 30 µg/m2/d. The pooled CR/CRi for these studies was 180/469 (38.37%). The most common hematological grade ≥3 adverse events were neutropenia 89/448 (19.87%), anemia 45/422 (10.70%), and thrombocytopenia 41/422 (9.7%).
A total of 509 patients were included in 5 observational studies. The overall CR/CRi reported was 129/509 (25.34%). Grade ≥3 neutropenia and thrombocytopenia were reported as 14/52(26.92%) in one study.CRS was reported in another study as 5/14 (35 %).
Combination regimens: In a phase II trial of Blinatumomab when given with a TKI (Dastanib), CR/CRi achieved was 27/47 (56.3%). Blinatumomab related neurotoxicity was observed in 2/12 (16.67%) patients. A total of 38 patients were included in 3 observational studies involving Blinatumomab and TKIs. The overall CR/CRi reported was 25/30(83.33%).
In a multicenter phase I/II trial blinatumomab when given with PD-1 inhibitor pembrolizumab (pembro) achieved a CR/CRi of 2/4 (50%). In a phase II study, Blinatumomab and PD-1 and CTLA-4 inhibitors were used in combination in 8 patients. The CR/CRi achieved was 4/5 (80%). Grade ≥3 non-hematological adverse events (AEs) were observed in 1/5 (20%) patients. Blinatumomab has been used in combination with Hyper-CVAD in a phase II study. The ORR and CR achieved have been 14/14 (100%) and 13/14 (93%) respectively. In a recent study, blinatumomab has been used along with miniHCVD + INO in 17 patients. The CR/CRi achieved was 13/17 (76.47%).
Conclusion:
Blinatumomab alone and in combination with TKIs, PD-1 inhibitors, CTLA-4 inhibitors,hyper-CVAD and miniHCVD + INO were well tolerated by adult patients with acute lymphocytic leukemia. Blinatumomab in combination therapy has shown superior efficacy in adult ALL patients as compared to blinatumomab monotherapy. Additional multicenter randomized, double-blind clinical trials are needed to confirm these results.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal